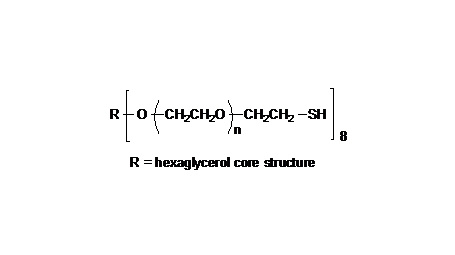产品中心
联系我们
销售专用:
地址:北京市海淀区西小口路66号中关村东升科技园C-1楼三层

- 产品描述
- 参考文献
-
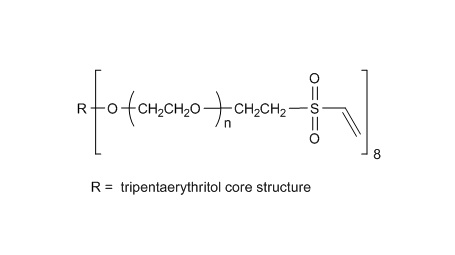
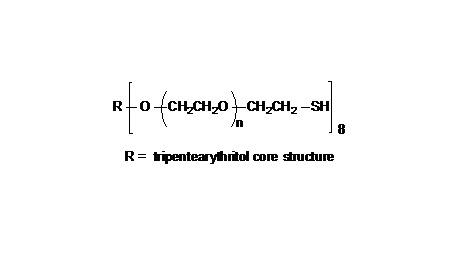
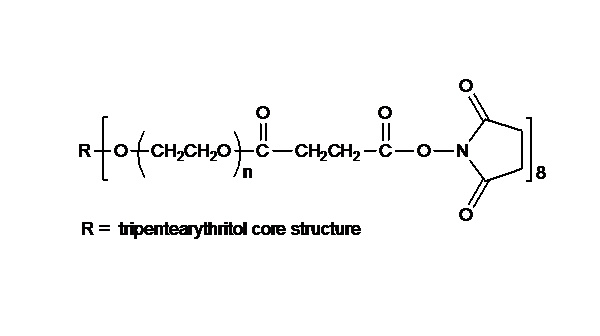
键凯科技提供高品质8ARM-NH2HCl-20K八臂聚乙二醇胺盐酸盐产品,产品取代率≥ 95%
键凯科技的8臂胺基产品可交联制备PEG水凝胶产品。PEG水凝胶在医疗器械和再生医学方面尤其是在药物的缓释控释,2维和3维细胞培养以及伤口的缝合和愈合方面有非常广泛的应用。键凯的8臂PEG原料来通过三聚季戊四醇和乙氧基聚合而成,每个PEG链的乙氧基单元数目不是完全相同的。键凯的多臂PEG产品的分子量指的是各臂分子量的总和。
键凯科技提供8ARM-NH2HCl分子量10000Da, 20000 Da,40000 Da产品 1克和10克包装。
键凯科技提供分装服务,需要收取分装费用,如果您需要分装为其他规格请与我们联系。
键凯科技同时提供其他分子量的8ARM-NH2HCl-20K产品,如你需要请与我司[email protected]联系。
键凯科技提供大批量生产产品及GMP级别产品,如需报价请与我们联系。
-
References:
- Schneider, M.C., et al., An In Vitro and In Vivo Comparison of Cartilage Growth in Chondrocyte-Laden Matrix Metalloproteinase-Sensitive Poly (Ethylene Glycol) Hydrogels with Localized Transforming Growth Factor β3, Acta biomaterialia, 2019.
- Armstrong, J.P., et al., Spatiotemporal quantification of acoustic cell patterning using Voronoï tessellation, Lab on a Chip, 2019.
- Rao, V.V., et al., Rescuing mesenchymal stem cell regenerative properties on hydrogel substrates post serial expansion, Bioengineering & translational medicine, 2019.
- Aziz, A.H., et al., The effects of dynamic compressive loading on human mesenchymal stem cell osteogenesis in the stiff layer of a bilayer hydrogel, Journal of tissue engineering and regenerative medicine, 2019.
- Aziz, A.H., et al., A comparison of hMSC osteogenesis in PEG hydrogels as a function of MMP‐sensitive crosslinker and crosslink density in chemically‐defined medium, Biotechnology and Bioengineering, 2019.
- Lee, J., et al., Glucose‐Responsive Trehalose Hydrogel for Insulin Stabilization and Delivery, Macromolecular bioscience, 2018, p.1700372.
- Carles-Carner, M., et al., The effects of hydroxyapatite nanoparticles embedded in a MMP-sensitive photoclickable PEG hydrogel on encapsulated MC3T3-E1 pre-osteoblasts, Biomedical Materials, 2018, 13(4), p.045009.
- Ovadia, E.M., et al., Designing well-defined photopolymerized synthetic matrices for three-dimensional culture and differentiation of induced pluripotent stem cells, Biomaterials science, 2018.
- Li, H., et al., Preparation of photo-responsive poly(ethylene glycol) microparticles and their influence on cell viability, Journal of Colloid and Interface Science, 2018, V. 514, P. 182-189.
- Tardy, B.L., et al., Formation of Polyrotaxane Particles via Template Assembly. Biomacromolecules, 2017.
- Guo, C., et al., Bio-orthogonal conjugation and enzymatically triggered release of proteins within multi-layered hydrogels, Acta Biomaterialia, 2017.
- Schneider, M.C., et al., Characterization of the chondrocyte secretome in photoclickable poly (ethylene glycol) hydrogels, Biotechnology and Bioengineering, 2017.
- Aziz, A.H., et al., Mechanical characterization of sequentially layered photo-clickable thiol-ene hydrogels, Journal of the Mechanical Behavior of Biomedical Materials, 2017, V. 65, p. 454-465.
- Braunger, J.A., et al., Interactions between circulating nanoengineered polymer particles and extracellular matrix components in vitro. Biomaterials Science, 2017.
- Liu, Y., et al., Trehalose Glycopolymer Enhances Both Solution Stability and Pharmacokinetics of a Therapeutic Protein, Bioconjugate Chemistry, 2017.
- Shih, H., Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine, Biomaterials Science, 2017, 5(3), 589-99.
- Chu, S., et al., Understanding the Spatiotemporal Degradation Behavior of Aggrecanase-Sensitive Poly (ethylene glycol) Hydrogels for use in Cartilage Tissue Engineering, Tissue Engineering, 2017.
- Shin, D.S., et al., Synthesis of microgel sensors for spatial and temporal monitoring of protease activity, ACS Biomaterials Science & Engineering, 2017.
- Jeon, O., et al., Dual-crosslinked hydrogel microwell system for formation and culture of multicellular human adipose tissue-derived stem cell spheroids. Journal of Materials Chemistry B, 2016, 4(20), 3526-33.
- Ma, Z., et al., Folate‐Conjugated Polylactic Acid–Silica Hybrid Nanoparticles as Degradable Carriers for Targeted Drug Delivery, On‐Demand Release and Simultaneous Self‐Clearance, ChemPlusChem, 2016.
- Maisonneuve, B. G. C., et al., Effects of Synthetic Biomacromolecule Addition on the Flow Behavior of Concentrated Mesenchymal Cell Suspensions, Biomacromolecules, 2015, 16(1), 275-283.
- Hennig, R., et al., Branched Polymer–Drug Conjugates for Multivalent Blockade of Angiotensin II Receptors, Molecular Pharmaceutics, 2015, 12 (9), 3292-3302.
- Skaalure, S.C., et al., An Enzyme-Sensitive PEG Hydrogel Based on Aggrecan Catabolism for Cartilage Tissue Engineering. Advanced Healthcare Materials, 2015, 4, 420–431.
- Sarfare, S., et al., Biocompatibility of a Synthetic Biopolymer for the Treatment of Rhegmatogenous
Retinal Detachment, J Clin Exp Ophthalmol, 2015, 6, 475. - Frith, J.E., et al., Effects of bound versus soluble pentosan polysulphate in PEG/HA-based hydrogels tailored for intervertebral disc regeneration. Biomaterials, 2014, 35(4), p. 1150-1162.
- Jeon, O., et al., Single and dual crosslinked oxidized methacrylated alginate/PEG hydrogels for bioadhesive applications. Acta Biomaterialia, 2014, 10(1), p. 47-55.
- McKinnon, D.D., et al., Design and Characterization of a Synthetically Accessible, Photodegradable Hydrogel for User-Directed Formation of Neural Networks, Biomacromolecules, 2014, 15, 2808−2816.
- Amoozgar, Z., et al., Dual-layer surface coating of PLGA-based nanoparticles provides slow-release drug delivery to achieve metronomic therapy in a paclitaxel-resistant murine ovarian cancer model, Biomacromolecules, 2014, 15(11), 4187-94.
- Chung, J., et al., Modular Multi-enzyme Cascade Process Using Highly Stabilized Enzyme Microbeads, Green Chem., 2014, 16, 1163-1167.
- McKinnon, D.D., Process Extension from Embryonic Stem Cell-Derived Motor Neurons through Synthetic Extracellular Matrix Mimics, Univ of Colorado at Boulder, 2014, 3635878.
- Cui, J., et al., Super-Soft Hydrogel Particles with Tunable Elasticity in a Microfluidic Blood Capillary Model, Advanced Materials, 2014, 26(43), 7295-7299.
- Gandavarapu, N. R., et al., Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity, Biomaterials Science, 2014, 2(3), 352-361.
- McKinnon, D.D., et al., Measuring cellular forces using bis-aliphatic hydrazone crosslinked stress-relaxing hydrogels, Soft Matter, 2014, 10, 9230.
- Strehin, I., et al., Hydrogels Formed by Oxo-ester Mediated Native Chemical Ligation, Biomater Sci., 2013, 1(6), 603–613.
- Barrett, D. G., et al., pH-Based Regulation of Hydrogel Mechanical Properties Through Mussel-Inspired Chemistry and Processing, Adv Funct Mater, 2013, 23(9), 1111-1119.
- Chung, J., et al., Magnetic-separable robust microbeads using a branched polymer for stable enzyme immobilization, Reactive and Functional Polymers, 2013, 73:1, P. 39-45.
- Jeon, O., et al., Regulation of Stem Cell Fate in a Three‐Dimensional Micropatterned Dual‐Crosslinked Hydrogel System, Advanced functional materials, 2013, 23.38, 4765-4775.
- Giorgi, M.E., et al., Improved bioavailability of inhibitors of Trypanosoma cruzi trans-sialidase: PEGylation of lactose analogs with multiarm polyethyleneglycol, Glycobiology, 2012, 22(10), p. 1363-1373.
- Zhou, J., et al., Real-time detection of implant-associated neutrophil responses using a formyl peptide receptor-targeting NIR nanoprobe, International Journal of Nanomedicine, 2012, 7 2057–2068.
- Christman, K.L., et al., Surface Patterning for Generating Defined Nanoscale Matrices, Stem Cells for Myocardial Regeneration, 2010, v. 660, p. 255-263.
- Tan, H., et al., Novel Multi-arm PEG-based Hydrogels for Tissue Engineering, Journal of biomedical materials research Part A., 2010, 92(3), 979-987.
- Schroeder, M. E., et al., Collagen networks within 3D PEG hydrogels support valvular interstitial cell matrix mineralization, Acta Biomaterialia, 2021, V. 119, P. 197-210.
- Schoonraad, SA, et al., The Effects of Stably Tethered BMP-2 on MC3T3-E1 Preosteoblasts Encapsulated in a PEG Hydrogel. Biomacromolecules. 2021, 22(3):1065-79.
- Caldwell, AS, et al, Mesenchymal stem cell‐inspired microgel scaffolds to control macrophage polarization. Bioengineering & Translational Medicine. 2021, 6(2):e10217.
- Song, J, et al, Influence of Poly (ethylene glycol) Molecular Architecture on Particle Assembly and Ex Vivo Particle–Immune Cell Interactions in Human Blood. ACS nano. 2021.
- Batan, D, et al., Hydrogel cultures reveal Transient Receptor Potential Vanilloid 4 regulation of myofibroblast activation and proliferation in valvular interstitial cells. The FASEB Journal. 2022.
- Yu, Y, et al., A 3D printed mimetic composite for the treatment of growth plate injuries in a rabbit model. NPJ Regenerative Medicine. 2022;7(1):1-4.
- Schroeder, M. E., et al., Osteopontin activity modulates sex‐specific calcification in engineered valve tissue mimics, Bioengineering & translational medicine 2023, 8.1, e10358.
-
Bhatta, R., et al., T cell-responsive macroporous hydrogels for in situ T cell expansion and enhanced antitumor efficacy, Biomaterials, V. 293, 2023.
产品询价