产品中心
联系我们
销售专用:
地址:北京市海淀区西小口路66号中关村东升科技园C-1楼三层

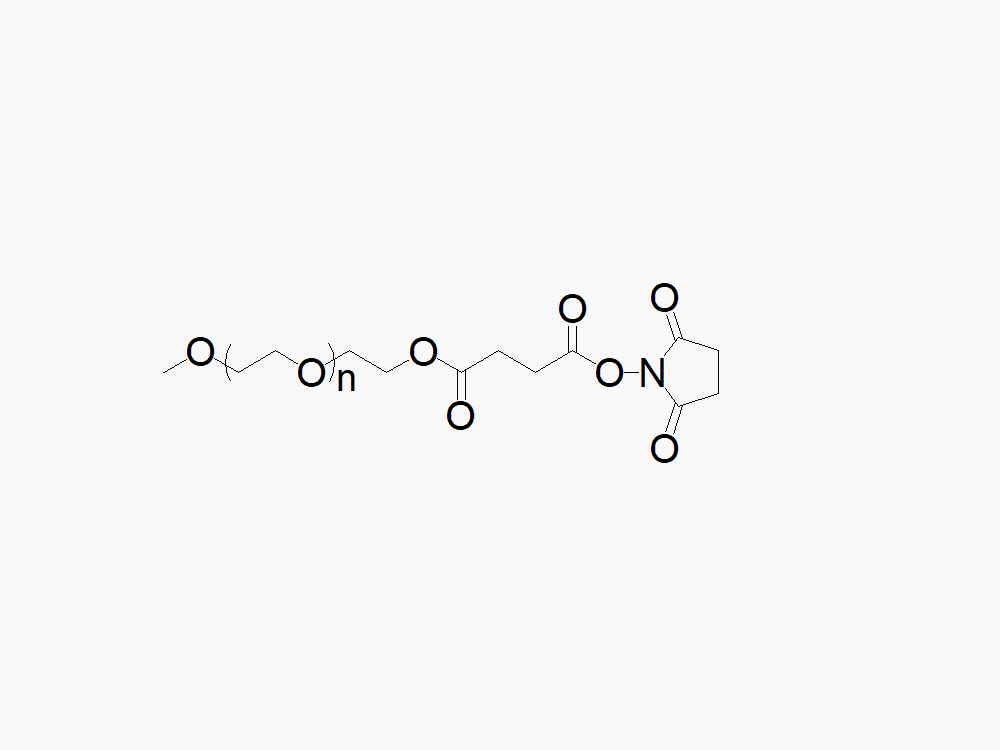
Methoxy PEG Succinimidyl Carboxymethyl Ester
产品代号:
M-PEG-SCM
产品纯度:
≥ 95%
包装规格:
1g, 10g, 100g等(特殊包装需收取分装费用)
分子量:
1000 Da-40000 Da,等
关键词:
所属分类:
科研客户小批量一键采购地址(小于5克)
- 产品描述
- 参考文献
-
甲氧基乙酯可以与药物或其他分子中的氨基进行反应,甲氧基聚乙二醇羧甲基琥珀酰亚胺酯中聚乙二醇连与NHS活性酯之间是通过醚键连接的,醚键是一种稳定的不可降解的化学键。在室温下PH=7-8的环境中本品可以快速与赖氨酸等分子中的氨基反应。
本品甲氧基聚乙二醇琥珀酰亚胺羧甲基酯的水解半衰期非常短,所以更能有效地选择具有空间结构优势的氨基基团进行聚乙二醇化。
键凯科技提供M-SCM分子量的1000 Da,2000 Da,5000 Da,10000 Da,2000 Da,30000 Da,35000 Da,40000 Da 的产品1克和10克包装。
键凯科技提供分装服务,需要收取分装费用,如果您需要分装为其他规格请与我们联系。
键凯科技同时提供其他分子量的M-SCM产品,如你需要请与我司[email protected]联系。
键凯科技提供大批量生产产品及GMP级别产品,如需报价请与我们联系。
-
References:
- Bisso, S., et al., Dual delivery of nucleic acids and PEGylated-bisphosphonates via calcium phosphate nanoparticles, European Journal of Pharmaceutics and Biopharmaceutics, 2019.
- Moussa, A., et al., Reducing-end “clickable” functionalizations of chitosan oligomers for the synthesis of chitosan-based diblock copolymers, Carbohydrate Polymers, 2019, V. 219, P. 387-394.
- Rajkumar, S., et al., Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy, Colloids and Surfaces B: Biointerfaces, 2019, V. 174, P. 252-259.
- Rompicharla, S.V., et al., Biotin functionalized PEGylated poly (amidoamine) dendrimer conjugate for active targeting of paclitaxel in cancer, International journal of pharmaceutics, 2019, 557:329-41.
- Feng, J., et al., Dendritic Polylysine Based ανβ3 Integrin Targeted Probe for Near-infrared Fluorescent Imaging of Glioma, Colloids and Surfaces B: Biointerfaces, 2019.
- Han, S., et al., A novel synergetic targeting strategy for glioma therapy employing borneol combination with angiopep-2-modified, DOX-loaded PAMAM dendrimer, Journal of drug targeting, 2018, 26(1), pp.86-94.
- Hu, Q., et al., Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage, Nanomedicine, 2018, 13(7), pp.749-767.
- Li, H., et al., Dendron‐Grafted Polylysine‐Based Dual‐Modal Nanoprobe for Ultra‐Early Diagnosis of Pancreatic Precancerosis via Targeting a Urokinase‐Type Plasminogen Activator Receptor, Advanced healthcare materials, 2018, 7(5), p.1700912.
- Zhang, L., et al., In vivo tumor active cancer targeting and CT-fluorescence dual-modal imaging with nanoprobe based on gold nanorods and InP/ZnS quantum dots, Journal of Materials Chemistry B, 2018.
- Badkas, A., et al., Modulation of in vitro phagocytic uptake and immunogenicity potential of modified Herceptin®-conjugated PLGA-PEG nanoparticles for drug delivery, Colloids and Surfaces B: Biointerfaces, 2018, V. 162, P. 271-278.
- Rajkumar, S., et al., Multi-functional nanocarriers based on iron oxide nanoparticles conjugated with doxorubicin, poly(ethylene glycol) and folic acid as theranostics for cancer therapy, Colloids and Surfaces B: Biointerfaces, 2018, V. 170, P. 529-537.
- Pan, H.M., et al., Engineering and Design of Polymeric Shells: Inwards Interweaving Polymers as Multilayer Nanofilm, Immobilization Matrix or Chromatography Resins. ACS Applied Materials & Interfaces, 2017.
- Liu, C., et al., iRGD-mediated core-shell nanoparticles loading carmustine and O6-benzylguanine for glioma therapy, Journal of Drug Targeting, 2017, 25(3):235-46.
- Gao, W., et al., Transferrin receptor-targeted pH-sensitive micellar system for diminution of drug resistance and targetable delivery in multidrug-resistant breast cancer, International Journal of Nanomedicine, 2017, 12:1047.
- Yang, C., et al., Effects of PEGylation on biomimetic synthesis of magnetoferritin nanoparticles, Journal of Nanoparticle Research, 2017, 19(3):101.
- Jiang, G., et al., Formulation of temozolomide-loaded nanoparticles and their targeting potential to melanoma cells, Oncology reports, 2017, 37(2):995-1001.
- Han, S., et al., A Novel Synergetic Targeting Strategy for Glioma Therapy Employing Borneol Combination with Angiopep-2-modified, DOX-loaded PAMAM Dendrimer, Journal of Drug Targeting, 2017, 1-20.
- Xu, L., et al., Oxidized catechol-derived poly (ethylene glycol) for thiol-specific conjugation. Reactive and Functional Polymers, 2017.
- Yu, Y., et al., A new NIR-triggered DOX and ICG co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy, Acta Biomaterialia, 2017.
- Ye, J., et al., High-Yield Synthesis of Monomeric LMWP (CPP)-siRNA Covalent Conjugate for Effective Cytosolic Delivery of siRNA, Theranostics, 2017, 7(9), 2495-2508
- Yuan, Z., et al., Multifunctional nanoparticles co-delivering EZH2 siRNA and etoposide for synergistic therapy of orthotopic non-small-cell lung tumor, Journal of Controlled Release, 2017, v. 268, P. 198-211.
- Alejo-Gonzalez, K., et al., PEGylation of cytochrome P450 enhances its biocatalytic performance for pesticide transformation, International Journal of Biological Macromolecules, 2017, v. 105:1, P. 163-170.
- Henley, W. H., et al., High resolution separations of charge variants and disulfide isomers of monoclonal antibodies and antibody drug conjugates using ultra-high voltage capillary electrophoresis with high electric field strength, Journal of Chromatography A, 2017, V. 1523, P. 72-79.
- Yu, Y., et al., A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy, Acta Biomaterialia, 2017, V. 59, P. 170-180.
- Cao, X., et al., Modulating the Cellular Immune Response of Oligonucleotides by Brush Polymer‐Assisted Compaction, Small, 2017, 13(43).
- Xu, Z., et al., A poly(amidoamine) dendrimer-based nanocarrier conjugated with Angiopep-2 for dual-targeting function in treating glioma cells, Polym. Chem., 2016, 7, 715-721.
- Dasargyri, A., et al., Findings questioning the involvement of Sigma-1 receptor in the uptake of anisamide-decorated particles, Journal of Controlled Release, 2016.
- Son, Y.J., et al., Electrospun Nanofibrous Sheets for Selective Cell Capturing in Continuous Flow in Microchannels, Biomacromolecules, 2016.
- Kumar, A.T., et al., Substrate-based near-infrared imaging sensors enable fluorescence lifetime contrast via built-in dynamic fluorescence quenching elements. ACS Sensors, 2016.
- Wang, W., et al., Doxorubicin-loaded pH-sensitive polymeric blends for synergistic cancer treatment, RSC Adv., 2016, 6, 31167-31176.
- Zhou, F., et al., Targeted delivery of microRNA-126 to vascular endothelial cells via REDV peptide modified PEG-trimethyl chitosan. Biomaterials science, 2016, 4(5):849-56.
- Li, F., et al., Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αvβ3 expression with an MRI modality in mice, Biomaterials, 2016, 102:162-74.
- Gao, X., et al., Nanoagonist-mediated endothelial tight junction opening: A strategy for safely increasing brain drug delivery in mice, Journal of Cerebral Blood Flow & Metabolism, 2016.
- Wu, D., et al., Phenylboronic acid-functionalized polyamidoamine-mediated Bcl-2 siRNA delivery for inhibiting the cell proliferation, Colloids and Surfaces B: Biointerfaces, 2016, 146:318-25.
- Rasson, A.S., et al., Filament Assembly by Spire: Key Residues and Concerted Actin Binding, Journal of Molecular Biology, 2015, 427 (4), p: 824-839.
- Jiao, Y., et al., Functionalized Hollow Mesoporous Silica Nanoparticles‐Based Controlled Dual‐Drug Delivery System for Improved Tumor Cell Cytotoxicity, Particle & Particle Systems Characterization, 2015, 32(2): 222-233.
- Oztug Durer, Z.A., et al., Metavinculin Tunes the Flexibility and the Architecture of Vinculin-Induced Bundles of Actin Filaments, Journal of Molecular Biology, 2015, 427:17, P. 2782-2798.
- Schmid, E.M., et al., Chapter 17 – Reconstitution of proteins on electroformed giant unilamellar vesicles, In: Methods in Cell Biology, Academic Press, 2015, 128, P. 319-338.
- Morry, J., et al., Dermal delivery of HSP47 siRNA with NOX4-modulating mesoporous silica-based nanoparticles for treating fibrosis, Biomaterials, 2015, 66.
- Fu, H., et al., Tumor‐Targeted Paclitaxel Delivery and Enhanced Penetration Using TAT ‐Decorated Liposomes Comprising Redox‐Responsive Poly(Ethylene Glycol), Journal of Pharmaceutical Science, 2015, 104(3), 1160-1173.
- McLeod, V. M., et al., Optimal PEGylation can Improve the Exposure of Interferon in the Lungs Following Pulmonary Administration, Journal of Pharmaceutical Sciences, 2015, 104(4), 1421-1430.
- Cheng, L., et al., Construction and evaluation of PAMAM–DOX conjugates with superior tumor recognition and intracellular acid-triggered drug release properties, Colloids and Surfaces B: Biointerfaces, 2015, 136, P. 37-45.
- Zheng, S., et al., Salvaging brain ischemia by increasing neuroprotectant uptake via nanoagonist mediated blood brain barrier permeability enhancement, Biomaterials, 2015, 66, P. 9-20.
- Paez, J.I., et al., Gauging and Tuning Cross-Linking Kinetics of Catechol-PEG Adhesives via Catecholamine Functionalization, Biomacromolecules, 2015, 16 (12), 3811-3818.
- Senanayake, T. H., et al., Nanogel-Conjugated Reverse Transcriptase Inhibitors and Their Combinations as Novel Antiviral Agents with Increased Efficacy against HIV-1 Infection, Molecular Pharmaceutics, 2015, 12 (12), 4226
- Yu, H., et al., Enzyme sensitive, surface engineered nanoparticles for enhanced delivery of camptothecin, Journal of Controlled Release, 2015, 216, P. 111-120.
- Zirbs, R., et al., Melt-grafting for the synthesis of core–shell nanoparticles with ultra-high dispersant density, Nanoscale, 2015, 7, 11216-11225.
- Chan, L.J., et al., PEGylation Does Not Significantly Change the Initial Intravenous or Subcutaneous Pharmacokinetics or Lymphatic Exposure of Trastuzumab in Rats but Increases Plasma Clearance after Subcutaneous Administration, Molecular Pharmaceutics, 2015, 12 (3), 794-809.
- Crouzier, T., et al., Modulating Mucin Hydration and Lubrication by Deglycosylation and Polyethylene Glycol Binding. Adv. Mater. Interfaces, 2015, 2.
- Wang, K., et al., Development of biodegradable polymeric implants of RGD-modified PEG-PAMAM-DOX conjugates for long-term intratumoral release, Drug Delivery, 2015, 22:3.
- Nannan, L., et al., Antitumor and antimetastatic effects of pemetrexed-loaded targeted nanoparticles in B16 bearing mice, Drug delivery, 2015, 1-9.
- Fahrländer E., et al., PEGylated human serum albumin (HSA) nanoparticles: preparation, characterization and quantification of the PEGylation extent, Nanotechnology, 2015, 26(14), 145103.
- Zhao, G., et al., Functional PEG–PAMAM-Tetraphosphonate Capped NaLnF4 Nanoparticles and their Colloidal Stability in Phosphate Buffer. Langmuir, 2014, 30(23): p. 6980-6989.
- Petek, N.A. and R.D. Mullins, Chapter Two – Bacterial Actin-Like Proteins: Purification and Characterization of Self-Assembly Properties, in Methods in Enzymology, D.V. Ronald, Editor., 2014, p. 19-34.
- Hu, W., et al., Redox and pH-responsive poly (amidoamine) dendrimer-poly (ethylene glycol) conjugates with disulfide linkages for efficient intracellular drug release, Colloids Surf B Biointerfaces, 2014, 123, p:254-63.
- Liu, S., et al., NIR initiated and pH sensitive single-wall carbon nanotubes for doxorubicin intracellular delivery, J. Mater. Chem. B, 2014, 2, 1125.
- Gonzalez, A. L., et al., Integrin-driven monocyte to dendritic cell conversion in modified extracorporeal photochemotherapy, Clin Exp Immunol, 2014, 175(3): 449-457.
- Chen, J., et al., A Facile Strategy for In Situ Controlled Delivery of Doxorubicin with a pH-Sensitive Injectable Hydrogel, Nano LIFE, 2014, 04:03.
- Wang, K., et al., Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates, Biomaterials, 2014, 35:30, p. 8735-8747.
- Qin, L., et al., A dual‑targeting liposome conjugated with transferrin and arginine‑glycine‑aspartic acid peptide for glioma‑targeting therapy, Oncology Letters, 2014, 8, 2000-2006.
- Yuan, W., et al., Increased Delivery of Doxorubicin Into Tumor Cells Using Extracellularly Activated TAT Functionalized Liposomes: In Vitro and In Vivo Study, Journal of Biomedical Nanotechnology, 2014, 10:8, p. 1563-1573(11).
- Zhou. J., et al., Enhanced and selective delivery of enzyme therapy to 9L-glioma tumor via magnetic targeting of PEG-modified, β-glucosidase-conjugated iron oxide nanoparticles, International Journal of Nanomedicine, 2014, 9:2905-2917.
- Saville, S. L., et al., The formation of linear aggregates in magnetic hyperthermia: Implications on specific absorption rate and magnetic anisotropy, Journal of Colloid and Interface Science, 2014, 424, P. 141-151.
- Kong, X., et al., A novel multifunctional poly(amidoamine) dendrimeric delivery system with superior encapsulation capacity for targeted delivery of the chemotherapy drug 10-hydroxycamptothecin. International Journal of Pharmaceutics, 2014, 465(1–2), p. 378-387.
- Si, Z., et al., pH-responsive near-infrared nanoprobe imaging metastases by sensing acidic microenvironment, RSC Adv., 2014, 4, 55548-55555.
- Mei, L., et al., Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. International Journal of Pharmaceutics, 2014, 468(1–2), p. 26-38.
- Tang, J., et al., Liposomes co-modified with cholesterol anchored cleavable PEG and octaarginines for tumor targeted drug delivery, Journal of Drug Targeting, 2014, 22:4.
- Zheng, S., et al., Multimodal Nanoprobes Evaluating Physiological Pore Size of Brain Vasculatures in Ischemic Stroke Models. Advanced Healthcare Materials, 2014, 3: 1909–1918.
- Gao, X., et al., Overcoming the blood–brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS nano, 2014, 8(4):3678-89.
- Wang, Q., et al., Comparative studies of salinomycin-loaded nanoparticles prepared by nanoprecipitation and single emulsion method, Nanoscale Research Letters, 2014, 9:351.
- Haiou Qu, et al., Controllable in Situ Synthesis of Magnetite Coated Silica-Core Water-Dispersible Hybrid Nanomaterials, Langmuir, 2013, 29(33) p: 10573–10578.
- Malik, R., et al., A single-layer peptide nanofiber for enhancing the cytotoxicity of trastuzumab (anti-HER), Journal of Nanoparticle Research, 2013, 15:1682.
- Giger, E. V., et al., siRNA transfection with calcium phosphate nanoparticles stabilized with PEGylated chelators, Adv Healthc Mater, 2013, 2(1): 134-144.
- Hansen, S.D, et al., Cytoplasmic actin: purification and single molecule assembly assays, InAdhesion Protein Protocols, 2013, p. 145-170.
- Gao, X., et al., Up-regulating blood brain barrier permeability of nanoparticles via multivalent effect, Pharmaceutical research, 2013, 30.10 : 2538-2548.
- Lei, Y., et al. Glutathione-sensitive RGD-poly(ethylene glycol)-SS-polyethylenimine for intracranial glioblastoma targeted gene delivery. J. Gene Med., 2013, 15: 291–305.
- Sabar, M. F., et al., Synthesis and Bioactivity Study of 30KDa Linear PEG-Interferon and its Comparison with Tri-Branched PEG-Interferon. J. Chem. Soc., 2013, 35.1: 119.
- Ran, R., et al., Enhanced tumor accumulation and cellular uptake of liposomes modified with ether-bond linked cholesterol derivatives, Die Pharmazie-An International Journal of Pharmaceutical Sciences, 2013, 68.8 : 668-674.
- Scott, M.A., Ultra-rapid 2-D and 3-D laser microprinting of proteins. Diss. Massachusetts Institute of Technology, 2013.
- Sun, C., et al., Bifunctional PEGylated exenatide-amylinomimetic hybrids to treat metabolic disorders: an example of long-acting dual hormonal therapeutics, Journal of medicinal chemistry, 2013, 56.22 : 9328-9341.
- Li, R., et al., Intelligently Targeted Drug Delivery and Enhanced Antitumor Effect by Gelatinase-Responsive Nanoparticles. PLoS ONE, 2013, 8(7): e69643.
- Keefe, A.J., et al., Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity, Nature Chemistry, 2012, 4, p: 59–63.
- Lee, G., et al., Real-Time Quantitative Monitoring of Specific Peptide Cleavage by a Proteinase for Cancer Diagnosis, Angew. Chem. Int. Ed., 2012, 51, 5837 –5841.
- Huang, G., et al., Dextran based pH-sensitive near-infrared nanoprobe for in vivo differential-absorption dual-wavelength photoacoustic imaging of tumors, J. Mater. Chem., 2012, 22, 22575.
- Doronin, K., et al., Construction of Targeted and Armed Oncolytic Adenoviruses, Methods in Molecular Biology, Oncolytic Viruses, 2012, 797.
- Qin, L., et al., Gelatinase-stimuli strategy enhances the tumor delivery and therapeutic efficacy of docetaxel-loaded poly (ethylene glycol)-poly (varepsilon-caprolactone) nanoparticles, Int J Nanomedicine 7, 2012, 281-295.
- Gillich, T., et al., Self-Assembly of Focal Point Oligo-catechol Ethylene Glycol Dendrons on Titanium Oxide Surfaces: Adsorption Kinetics, Surface Characterization, and Nonfouling Properties, J.A.C.S., 2011, 133(28), p: 10940–10950.
- Wang, Y.Y., et al., Introducing RGD Peptides on PHBV Films through PEG-Containing Cross-Linkers to Improve the Biocompatibility, Biomacromolecules, 2011, 12(3), p: 551–559.
- Soontornworajit, B., et al., Affinity hydrogels for controlled protein release using nucleic acid aptamers and complementary oligonucleotides, Biomaterials, 2011, 32(28): 6839-6849.
- Zhang, L., et al., RGD-modified PEG–PAMAM–DOX conjugates: In vitro and in vivo studies for glioma, European Journal of Pharmaceutics and Biopharmaceutics, 2011, 79:2, P. 232-240.
- Lu, Z-X, et al., Development of small interfering RNA delivery system using PEI-PEG-APRPG polymer for antiangiogenic vascular endothelial growth factor tumor-targeted therapy, International Journal of Nanomedicine, 2011, 6:1661-1673.
- Kuai, R., et al., Targeted Delivery of Cargoes into a Murine Solid Tumor by a Cell-Penetrating Peptide and Cleavable Poly(ethylene glycol) Comodified Liposomal Delivery System via Systemic Administration, Mol. Pharmaceutics, 2011, 8(6), p: 2151–2161.
- Chen, H., et al., High-level production of uricase containing keto functional groups for site-specific PEGylation, Biochemical Engineering Journal, 2011, 58–59, p. 25-32.
- Zhu, S., et al., PEGylated PAMAM Dendrimer-Doxorubicin Conjugates: In Vitro Evaluation and In Vivo Tumor Accumulation, Pharmaceutical Research, 2010, 27:1, p. 161-174.
- Georgianna, W.E., et al., Photocleavable Polyethylene Glycol for the Light-Regulation of Protein Function, Bioconjugate Chem., 2010, 21(8) p: 1404–1407.
- Zhu, S., et al., Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: the effects of PEGylation degree and drug conjugation style, Biomaterials, 2010, 31(6):1360-71.
- Huang, R., et al., Evaluation and mechanism studies of PEGylated dendrigraft poly-L-lysines as novel gene delivery vectors, Nanotechnology, 2010, 21:26.
- Kuai, R., et al., Efficient Delivery of Payload into Tumor Cells in a Controlled Manner by TAT and Thiolytic Cleavable PEG Co-Modified Liposomes, Molecular Pharmaceutics, 2010, 7 (5), 1816-1826.
- Liu, Q., et al., Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles, Biomaterials, 2013, 34:29, p. 7191-7203.
- Zhang, H., et al., Efficient Transfection of Blood−Brain Barrier Endothelial Cells by Lipoplexes and Polyplexes in the Presence of Nuclear Targeting NLS-PEG-Acridine Conjugates, Bioconjugate Chemistry, 2009, 20(1), 120-128.
- Doronin, K., et al., Chemical Modification with High Molecular Weight Polyethylene Glycol Reduces Transduction of Hepatocytes and Increases Efficacy of Intravenously Delivered Oncolytic Adenovirus, Human Gene Therapy, 2009, 20(9): 975-988.
- Zhang, H., et al., Efficient Nuclear Targeting by NLS-PEG-Acridine Conjugates in the Transfection of Blood-Brain Barrier Endothelial Cells with Lipoplexes and Polyplexes, Bioconjugate chemistry, 2009, 20(1):120-128.
- Cao, J., et al., Quantitative determination of pegylated consensus interferon in rhesus monkey serum using a competitive enzyme-linked immunosorbent assay, Immunopharmacology and Immunotoxicology, 2009, 31:4.
- Ochs, C.J., et al., Low-Fouling, Biofunctionalized, and Biodegradable Click Capsules, Biomacromolecules, 2008, 9(12), p: 3389–3396.
- Weaver, E.A., et al., Effects of Shielding Adenoviral Vectors with Polyethylene Glycol on Vector-Specific and Vaccine-Mediated Immune Responses, Human Gene Therapy, 2008, 19:1369–1382.
- Hofherr, S.E., et al., Modification of Adenoviral Vectors With Polyethylene Glycol Modulates In Vivo Tissue Tropism and Gene Expression, Molecular Therapy, 2008, 16 7.
- Sousa, A., et al., Design of experiments to select triphenylphosphonium-polyplexes with suitable physicochemical properties for mitochondrial gene therapy, Journal of Molecular Liquids, 2020, V. 302.
-
Yan, J., et al., Tumor Contrast Imaging with Gas Vesicles by Circumventing the Reticuloendothelial System, Ultrasound in Medicine & Biology, 2020, V. 46(2); P. 359-368.
-
Sousa, A., et al., Design of experiments to select triphenylphosphonium-polyplexes with suitable physicochemical properties for mitochondrial gene therapy, Journal of Molecular Liquids, 2020; 302.
-
Chen, L., Ultra-small MoS2 nanodots-incorporated mesoporous silica nanospheres for pH-sensitive drug delivery and CT imaging, Journal of Materials Science & Technology, 2020.
-
Amatya, R, et al., In Vitro and In Vivo Evaluation of PEGylated Starch-Coated Iron Oxide Nanoparticles for Enhanced Photothermal Cancer Therapy. Pharmaceutics. 2021, 13(6):871.
-
Fu, D, et al., A novel redox-responsive ursolic acid polymeric prodrug delivery system for osteosarcoma therapy. Drug Delivery. 2021, 28(1):195-205.
-
Gao, M, et al., A Manganese Phosphate Nanocluster Activates the cGAS‐STING Pathway for Enhanced Cancer Immunotherapy. Advanced Therapeutics. 2021, 2100065.
-
Sheng, Q, et al., Comprehensively enhanced delivery cascade by transformable beaded nanofibrils for pancreatic cancer therapy. Nanoscale. 2021.
-
Godinho, B.M., et al., PK Modifying Anchors Significantly Alter Clearance Kinetics, Tissue Distribution and Efficacy of Therapeutics siRNAs, Molecular Therapy-Nucleic Acids, 2022.
-
Xu, K., et al., Injectable host-guest gel nanovaccine for cancer immunotherapy against melanoma. Materials Today Advances, 2022, 15.
-
Fan, N., et al., Preparation of an HI-6-loaded brain-targeted liposomes based on the nasal delivery route and the evaluation of its reactivation of central toxic acetylcholinesterase, European Journal of Pharmaceutical Sciences, 184, 2023.
-
Lin, Z., et al., Hypoxia-induced cysteine metabolism reprogramming is crucial for the tumorigenesis of colorectal cancer, Redox Biology, 2024. Keywords: Colorectal cancer; Cysteine/Cystine; Transporter genes; ATF4; Hypoxia; ROS homeostasis; methoxy PEG succinimidyl carboxymethyl ester of MW 5000 (PEG-5K)
产品询价