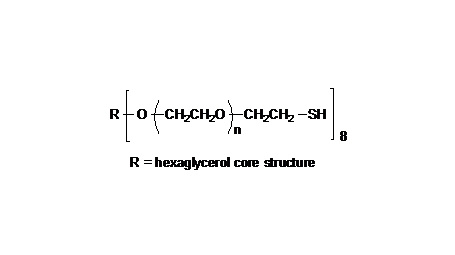产品中心
联系我们
销售专用:
地址:北京市海淀区西小口路66号中关村东升科技园C-1楼三层

- 产品描述
- 参考文献
-
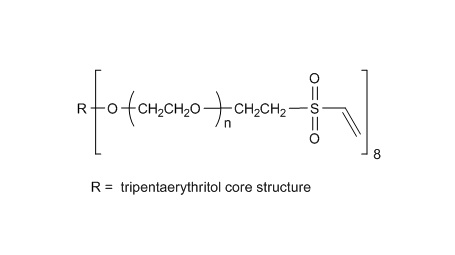
键凯科技提供高品质四臂聚乙二醇马来酰亚胺产品,产品取代率>90%。
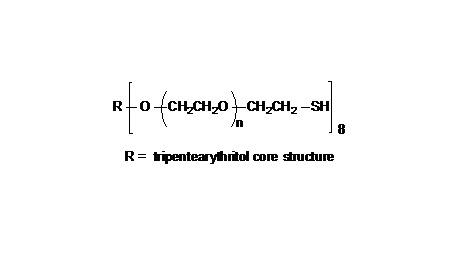
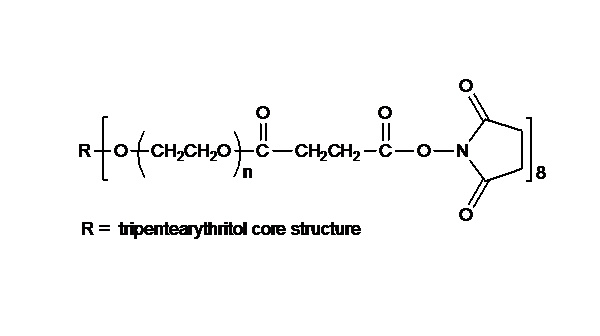
键凯科技的4臂马来酰亚胺产品可用来交联制备PEG水凝胶产品。PEG水凝胶在医疗器械和再生医学方面尤其是在药物的缓释控释,2维和3维细胞培养以及伤口的缝合和愈合方面有非常广泛的应用。键凯的4臂PEG原料来源于季戊四醇和环氧乙烷聚合而成,每个PEG链的乙氧基单元数目不是完全相同的。键凯的多臂PEG产品的分子量指的是各臂分子量的总和。
键凯科技提供4ARM-MAL-20K分子量10000Da, 20000 Da,40000 Da产品 1克和10克包装。
键凯科技提供分装服务,需要收取分装费用,如果您需要分装为其他规格请与我们联系。
键凯科技同时提供其他分子量的4ARM-MAL产品,如你需要请与我司[email protected]联系。
键凯科技提供大批量生产产品及GMP级别产品,如需报价请与我们联系。
-
References:
- Li, H., et al., Synthesis of thiol-terminated PEG-functionalized POSS cross-linkers and fabrication of high-strength and hydrolytic degradable hybrid hydrogels in aqueous phase, European Polymer Journal, 2019, 116:74-83.
- Atallah, P., et al., Charge-tuning of glycosaminoglycan-based hydrogels to program cytokine sequestration, Faraday Discussions, 2019.
- Dai, J., et al., Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1α loaded proteolytically degradable hydrogel for recellularization and remodeling, Acta biomaterialia, 2019.
- Tunn, I., et al., Bioinspired Histidine–Zn2+ Coordination for Tuning the Mechanical Properties of Self-Healing Coiled Coil Cross-Linked Hydrogels, Biomimetics, 2019, 4(1):25.
- Jansen, L.E., et al., Control of thiol-maleimide reaction kinetics in PEG hydrogel networks, Acta Biomaterialia, 2018, V. 70, P. 120-128.
- Schweikle, M., et al.,. Injectable synthetic hydrogel for bone regeneration: Physicochemical characterisation of a high and a low pH gelling system, Materials Science and Engineering: C, 2018, 90, pp.67-76.
- Brooks, E.A., et al., Complementary, Semi-automated Methods for Creating Multi-dimensional, PEG-based Biomaterials, ACS Biomaterials Science & Engineering, 2018.
- Matsumura, K., et al., Urokinase injection-triggered clearance enhancement of a 4-arm PEG-conjugated 64 Cu-bombesin analog tetramer: A novel approach for the improvement of PET imaging contrast, International journal of pharmaceutics, 2018.
- Yang, T., et al., Superparamagnetic colloidal chains prepared via Michael-addition, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, V. 540, P. 23-28.
- Shen, J., et al., Hydrolytically degradable POSS-PEG hybrid hydrogels prepared in aqueous phase with tunable mechanical properties, swelling ratio and degradation rate, Reactive and Functional Polymers, 2018, V. 123, P. 91-96.
- Day, J.R., et al., The impact of functional groups of poly(ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host, Acta Biomaterialia, 2018, Vol. 67, P. 42-52.
- Tondera, C., et al., In Vivo Examination of an Injectable Hydrogel System Crosslinked by Peptide–Oligosaccharide Interaction in Immunocompetent Nude Mice, Advanced Functional Materials, 2017, 27(15).
- Maitz, M.F., et al., Adaptive release of heparin from anticoagulant hydrogels triggered by different blood coagulation factors, Biomaterials, 2017, 135:53-61.
- Robinson, K.G., et al., Reduced Arterial Elasticity due to Surgical Skeletonization is Ameliorated by Abluminal PEG Hydrogel, Bioengineering & Translational Medicine, 2017.
- Bas, O., et al., Biofabricated soft network composites for cartilage tissue engineering. Biofabrication, 2017.
- Nowak, M., et al., Modular GAG-matrices to promote mammary epithelial morphogenesis in vitro, Biomaterials 2017, 112, p. 20-30.
- Hesse, E., et al., Peptide‐functionalized starPEG/heparin Hydrogels Direct Mitogenicity, Cell Morphology and Cartilage Matrix Distribution in vitro and in vivo. Journal of Tissue Engineering and Regenerative Medicine, 2017.
- Gencoglu, M.F., et al., Comparative study of multicellular tumor spheroid formation methods and implications for drug screening. ACS Biomaterials Science & Engineering, 2017.
- Skoumal, M.J, Localized Tolerance and Development of an Alternative Transplant Site to Treat Type 1 Diabetes, University of Michigan, 2017.
- Wu, F., et al., A novel synthetic microfiber with controllable size for cell encapsulation and culture. Journal of Materials Chemistry B, 2016, 4(14):2455-65.
- Taubenberger, A.V., et al., 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments, Acta biomaterialia, 2016.
- Darling, N.J., et al., Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly (ethylene glycol) hydrogels, Biomaterials, 2016.
- Danmark, S., et al., Tailoring Supramolecular Peptide-Poly (ethylene glycol) Hydrogels by Coiled Coil Self-Assembly and Self-Sorting. Biomacromolecules, 2016.
- Rios, P.D., et al., Mold‐casted non‐degradable, islet macro‐encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice. Biotechnology and bioengineering, 2016.
- Wang, J.J, et al., Biomimetic synthesis of platelet-shaped hydroxyapatite mesocrystals in a collagen mimetic peptide–PEG hybrid hydrogel, Materials Letters, 2015, 159, P. 150-153.
- Mahadevaiah, S., et al., Decreasing matrix modulus of PEG hydrogels induces a vascular phenotype in human cord blood stem cells, Biomaterials, 2015, 62, p. 24-34.
- Griffin, D. R., et al., Hybrid Photopatterned Enzymatic Reaction (HyPER) for In situ Cell Manipulation, Chembiochem : a European journal of chemical biology, 2014, 15(2): 233-242.
- Liang, Y., et al., Multifunctional lipid-coated polymer nanogels crosslinked by photo-triggered Michael-type addition, Polym. Chem., 2014, 5, 1728-1736.
- Maitz, M.F., et al., Bio-responsive polymer hydrogels homeostatically regulate blood coagulation, Nat Commun, 2013, 4.
- Robinson, K. G., et al., Differential effects of substrate modulus on human vascular endothelial, smooth muscle, and fibroblastic cells, Journal of Biomedical Materials Research, 2012, 100(5): 1356-1367.
- Lu, H.D., et al., Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism, Biomaterials, 2012, 33(7): p. 2145-2153.
- Soon, A.S.C., et al., Modulation of fibrin matrix properties via knob:hole affinity interactions using peptide–PEG conjugates, Biomaterials, 2011, 32:19, P. 4406-4414.
- Nie, T., et al., Production of heparin-containing hydrogels for modulating cell responses, Acta Biomaterialia, 2009, 5(3), p: 865-875.
- Schirmer, L., et al., Glycosaminoglycan-based hydrogels with programmable host reactions, Biomaterials, 2020, V. 228.
- Wang, J., et al., An injectable PEG hydrogel controlling neurotrophin-3 release by affinity peptides,Journal of Controlled Release, 2021, 330, P. 575-586.
- Scott, R. A., et al., Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages, Acta Biomaterialia, 2021, V. 122, P. 220-235.
- Cheng, L., et al., Bioresponsive micro-to-nano albumin-based systems for targeted drug delivery against complex fungal infections. Acta Pharmaceutica Sinica B. 2021
- Liu, S, et al., Injectable and Degradable PEG Hydrogel with Antibacterial Performance for Promoting Wound Healing. ACS Applied Bio Materials. 2021, 4(3):2769-80.
- Guo, R, et al., Anticalcification Potential of POSS-PEG Hybrid Hydrogel as a Scaffold Material for the Development of Synthetic Heart Valve Leaflets. ACS Applied Bio Materials. 2021, 4(3):2534-43.
- Kim, J, et al., In Situ Crosslinked Hydrogel Depot for Sustained Antibody Release Improves Immune Checkpoint Blockade Cancer Immunotherapy. Nanomaterials. 2021, 11(2):471.
-
Xu, Y., et al., A self-assembled dynamic extracellular matrix-like hydrogel system with multi-scale structures for cell bioengineering applications, Acta Biomaterialia, V. 162, 2023, P. 211-225.
-
Martin, K. E., et al., Hydrolytic hydrogels tune mesenchymal stem cell persistence and immunomodulation for enhanced diabetic cutaneous wound healing, Biomaterials, 301, 2023.
-
Ilochonwu, B. C., et al., Thermo-responsive Diels-Alder stabilized hydrogels for ocular drug delivery of a corticosteroid and an anti-VEGF fab fragment, Journal of Controlled Release, 361, 2023.
-
Laura A. Milton, L.A., et al., Liver click dECM hydrogels for engineering hepatic microenvironments, Acta Biomaterialia, 2024. Keywords: Decellularized extracellular matrix; Liver; Michael-type addition; Hydrogel; 3D cell culture; 4-arm PEG-SH; 4-arm PEG-MAL
产品询价